The hype and hope of new food allergy treatments - Prof Perrett writes for Nature Medicine
- Published
- Monday, April 22, 2024 - 7:00 PM
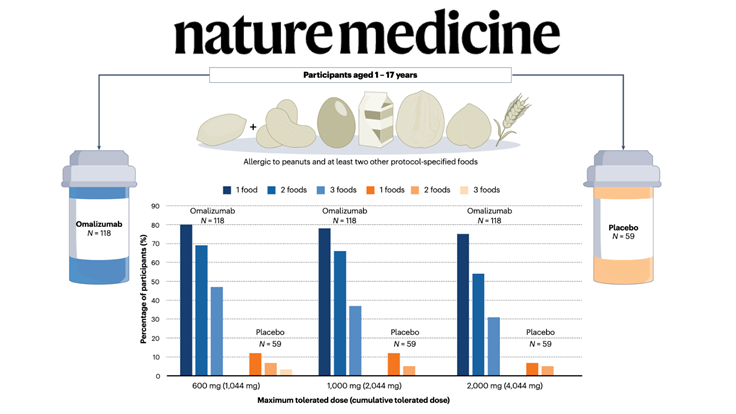
In a recent issue of The New England Journal of Medicine, Wood et al. report results of the first stage (of three) of the OUtMATCH trial, which evaluated the monoclonal antibody omalizumab (anti-IgE) as monotherapy in children and adults with multiple food allergies.
On the basis of these results, the US Food and Drug Administration (FDA) approved the expanded use of omalizumab in children (from 1 year of age) and adults with IgE-mediated food allergy, making it the first medication approved to help reduce the risk of severe allergic reactions across multiple foods.
Professor Kirsten Perrett, Centre for Food Allergy Research Director and Murdoch Children's Research Institute Population Allergy Group Lead, writes for Nature Medicine about the hype and hope of new food allergy treatments.
Read more in Nature Medicine
Want to take part in food allergy research?
Visit the Allergy Studies Directory and receive email alerts when new studies are listed.
Become a CFAR member
Are you part of the Australian and New Zealand food allergy research community?










